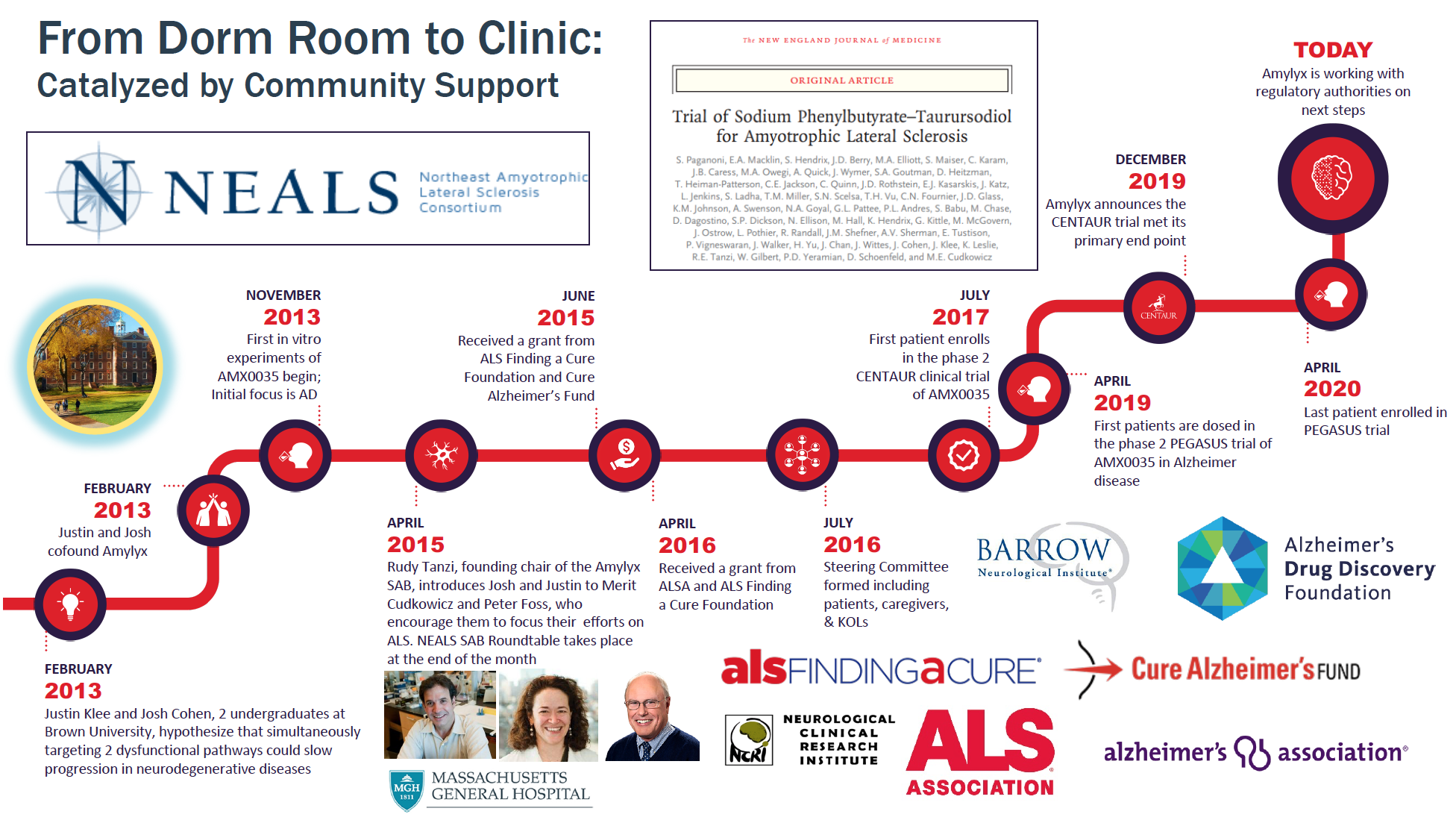
ALSFAC-Funded Project Announces Successful ALS Clinical Trial and High-Profile Publication
Amylyx, a start-up biotech company developing new treatments for ALS supported by ALS Finding a Cure®, and Mass General Hospital, announced the publication of results from its successful pivotal stage ALS clinical trial in the prestigious New England Journal of Medicine (NEJM). ALS Finding A Cure® provided two grants to Amylyx: the first in collaboration with Cure Alzheimer’s Disease to support required preclinical toxicology studies, and the second to fund this trial in collaboration with The ALS Association as part of the ALS ACT initiative.
The clinical trial, known as CENTAUR, was a 24-week, randomized, double-blind, placebo-controlled Phase 2/3 clinical trial of 137 individuals with ALS, and was conducted across 25 top medical centers in the U.S. The trial demonstrated that treatment with the Amylyx drug, AMX0035, was well tolerated and decreased the rate of decline, as assessed by the the ALS Functional Rating Scale-Revised (ALSFRS-R), compared to placebo in people with ALS. The trial was led by Drs. Sabrina Paganoni and Merit Cudkowicz of The Healey and AMG Center for ALS at Massachusetts General Hospital, ALS Finding a Cure® investigator and Chief Medical Officer respectively.
The study met its primary endpoint and showed the following:
- After 24 weeks, patients treated with AMX0035 scored on average 2.92 points higher at the end of 24 weeks of follow up (p=0.01). o The ALSFRS-R is a 48-point questionnaire measuring daily functions such as the ability to walk, dress independently, self-feed, speak and breathe.
- o A 1-2 point change in the ALSFRS-R score can indicate a significant reduction in a patient’s ability to function independently. Some examples of a two-point change on this scale include the difference between an individual eating successfully with some difficulty vs needing a feeding tube, or walking with assistance versus not walking at all.
- In line with the primary outcome, patients on AMX0035 also showed some benefits on measures of muscle strength, breathing and hospitalization frequency.
- Overall, safety was comparable between the groups, with fewer serious adverse events in the active group as compared to the placebo group (9 out of 48 patients (19%) in placebo vs 11 out of 89 patients (12%) in the AMX0035 group).
Participants who completed the CENTAUR study were given the option after the trial to enroll in an open-label extension study and receive AMX0035 long-term. 92% of eligible CENTAUR participants elected to enroll in the extension study. Interim data from the ongoing open-label extension study are being submitted to a peer-reviewed journal shortly and will be published in the coming months.
More information on the CENTAUR trial can be found at www.clinicaltrials.gov, NCT03127514.